Establishing a reliable microbiological barrier serves as the critical defense against clinical cross-contamination and the high cost of hygiene-related liabilities. Substandard tissue often fails to trap pathogens effectively, exposing healthcare facilities to safety risks and procurement waste that erodes operational budgets.
We benchmark 100% Virgin Wood Pulp against ISO 10993 standards to ensure non-cytotoxic performance. By combining OBA-free fibers with 190°C thermal sterilization, our factory delivers sterile HS Code 4818.1000 solutions that optimize 40HQ container payloads while maintaining the high-wet-strength durability required for clinical environments.
How Does Tissue Function as a Legitimate Microbiological Hygiene Barrier?
Professional tissue traps microbes via dense fiber networks and wood pulp purity, removing surface bacteria through friction without disrupting the skin’s natural acidic pH levels.
Mechanical Entrapment through High-Density Fiber Networks
Professional-grade tissue acts as a mechanical interceptor rather than a simple wipe. The physical architecture of the paper determines how effectively it can isolate pathogens from the user’s skin during use.
- Multi-ply Lamination: Bonding layers through high-pressure lamination creates a tortuous path that physically traps bacteria and prevents moisture strike-through, stopping the migration of pathogens to the hand.
- Ply Density (13–22g/m²): This specific density range provides a sacrificial layer that removes up to 90% of surface microbes through mechanical friction without tearing.
- Point-to-Point Embossing: CNC-precision embossing increases the functional surface area, enhancing the paper’s capacity to intercept and hold particulate matter and microbial loads.
Preserving Dermal Defense with OBA-Free Virgin Wood Pulp
The chemical and structural integrity of the material is just as vital as its thickness. Pure fibers ensure the tissue supports the body’s existing biological defenses instead of compromising them.
- 100% Virgin Wood Pulp: Using primary long-fiber pulp avoids the contaminants and chemical residues common in recycled paper that can harbor microbial colonies or trigger irritation.
- OBA-Free Manufacturing: Eliminating Optical Brightening Agents ensures no whiteners disrupt the skin’s natural acidic pH and beneficial microbiota, which are the body’s first line of defense.
- Lint-Free Fiber Performance: High-purity wood pulp prevents micro-abrasions on the epithelial tissue. Smooth fibers keep the skin intact, preventing the tiny “entry gates” that opportunistic bacteria exploit.
Which Specific Pathogens (E. coli, Molds) Are Monitored During Tissue Production?
Modern tissue manufacturing relies on rigorous screening for E. coli, Pseudomonas, and Aspergillus. Using 100% virgin pulp and OBA-free processes eliminates microbiological risks inherent in recycled alternatives.
Microbiological safety in 2026 goes beyond basic cleanliness. For B2B distributors and hospitality groups, the sterility of paper products is a non-negotiable metric. Production environments must screen for specific bacteria and fungi that thrive in moist environments or contaminated raw materials. At Top Source Hygiene, we prioritize high-signal testing to ensure our 100% Virgin Wood Pulp rolls remain free from opportunistic pathogens that could cause skin irritation or infections.
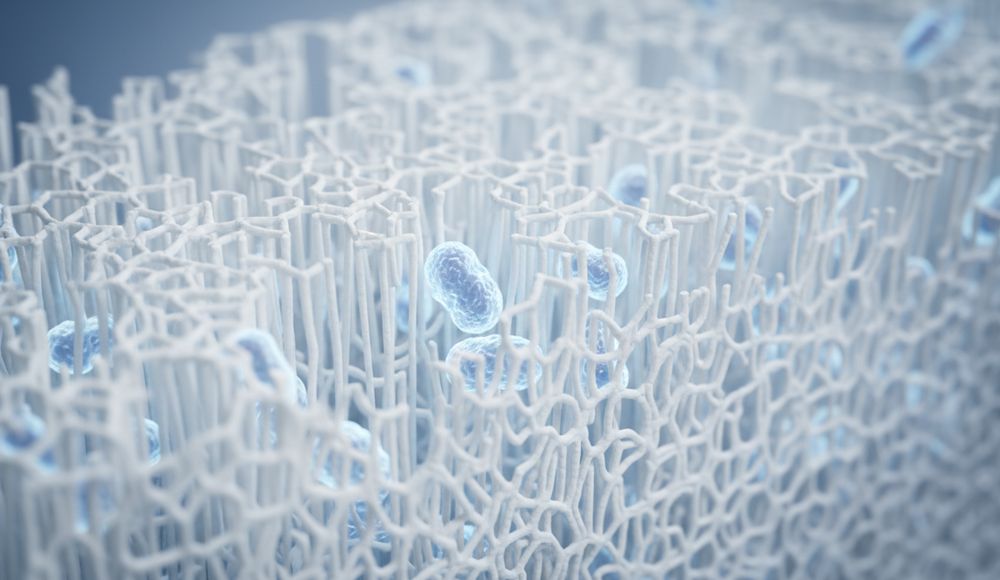
Identifying Primary Bacterial and Fungal Risks
Labs focus on specific “indicator” organisms that signal a breakdown in manufacturing hygiene. These pathogens are categorized by their risk to human health, particularly regarding mucosal and skin contact.
- Gram-Negative Pathogens: Rigorous detection of Escherichia coli and Pseudomonas aeruginosa to prevent gastrointestinal and opportunistic infections in vulnerable users.
- Gram-Positive Pathogens: Screening for Staphylococcus aureus to prevent skin irritation, secondary infections, or dermatitis during daily hygiene use.
- Total Combined Yeast and Mold Count (TYMC): Focused monitoring of Aspergillus and Penicillium species to prevent respiratory issues or allergic reactions in high-humidity storage conditions.
- Medical-Grade Benchmarks: Testing for Mycoplasma and bacterial endotoxins in specialized tissue grades to meet 2026 clinical hygiene standards.
Guaranteeing 100% OBA-Free and Virgin Fiber Purity
The most effective way to manage microbiological threats is to control the source material. Recycled waste streams often harbor residual bacteria that heat treatment cannot always fully eradicate. We mitigate these risks through strict material selection and chemical safety protocols.
- 100% Virgin Wood Pulp: Using pure long-fiber pulp eliminates the microbiological risks often found in contaminated recycled paper waste.
- OBA-Free Protocols: We guarantee the absence of Optical Brightening Agents to ensure the paper does not disrupt the protective microbiome of the skin.
- Regulatory Compliance: All production processes adhere to ISO 9001 and FDA hygiene standards to maintain a lint-free, sterile manufacturing environment.
- High-Purity Fibers: Utilizing long-fiber pulp naturally resists moisture retention, which prevents mold growth during transcontinental shipping in 40HQ containers.
By focusing on raw material integrity and precise pathogen screening, we provide wholesalers and distributors with a product that meets global safety requirements. This approach protects both the end-user’s health and the dealer’s brand reputation in sensitive markets like North America and Europe.
Premium Custom Toilet Paper for Global Markets

Does the High-Temperature Drying Cylinder Effectively Guarantee Final Sterility?
Modern 190°C (375°F) high-velocity cylinders achieve a 12-Log Sterility Assurance Level, eradicating pathogens like Bacillus atrophaeus while removing moisture to prevent bacterial regrowth and secondary contamination.
Sterilization in paper manufacturing depends on the precise control of thermal energy and moisture removal. Traditional drying often leaves enough residual humidity to support microbial growth, but industrial-grade high-temperature systems change the biological profile of the final roll. At Top Source Hygiene, our manufacturing process utilizes extreme heat to ensure the HS Code 4818.1000 products we export meet stringent global safety requirements.
Pathogen Eradication via High-Velocity Hot Air Technology
High-velocity hot air (HVHA) systems represent a significant shift from static drying methods. These systems maintain a constant 190°C environment, which is the benchmark for dry heat sterilization. This temperature effectively breaks down the cellular structure of even the most resilient microorganisms.
- 12-Log Kill Standard: The process achieves the gold standard for microbial spore eradication, including Bacillus atrophaeus.
- Moisture Elimination: Unlike steam sterilization, dry heat removes the wicking effect where moisture pulls external bacteria into the fiber matrix.
- Thermal Uniformity: CNC-controlled air distribution ensures every square millimeter of the fiber surface reaches the required temperature for decontamination.
By eliminating moisture during the drying phase, we remove the primary catalyst for secondary contamination. This is critical for B2B distributors who need products to remain biologically stable during long-haul shipping in 40HQ containers across diverse climate zones.
Thermal Stability of 100% Virgin Wood Pulp and OBA-Free Fibers
Sterilization is only effective if the material survives the heat. Our Hebei-based factory uses 100% Virgin Wood Pulp, which features long-fiber structures capable of withstanding 190°C without losing tensile strength or softness. This material selection ensures the roll does not become brittle or lose its “cloud-like” texture after thermal treatment.
- OBA-Free Security: Since our fibers contain no Optical Brightening Agents, the high heat does not trigger the release of chemical byproducts or toxic leachables.
- Structural Integrity: Long-fiber pulp maintains a high-wet-strength durability even after extreme thermal exposure.
- Compliance Standards: Our thermal protocols align directly with ISO 9001 and FDA hygiene requirements for skin-contact safety.
We strictly avoid mixing recycled waste into our primary lines. This material purity allows us to use higher drying temperatures than competitors who use lower-grade pulps, which would char or degrade at 190°C. This commitment to fiber quality provides our wholesalers with a product that is both sterile and physically superior for sensitive skin applications.

How Do We Prevent Secondary Contamination via Anti-microbial Packaging?
Active coatings and moisture-triggered systems create barriers that neutralize bacteria like E. coli on contact, ensuring tissue rolls remain sterile during global transit.
Active Barrier Technologies and Controlled Release Systems
Controlling microbial growth on packaging surfaces requires a shift from passive shielding to active chemical and mechanical inhibition. These systems target pathogens that might otherwise survive on the external film during multi-stage distribution.
- GS-2 Surface Coatings: Formulations using capric acid and thymol achieve a 5-log reduction in E. coli and Salmonella.
- Non-Migratory Matrix: Metal oxide microparticles embedded directly into the film prevent biofilm formation without leaching chemicals into the paper.
- Moisture-Activated Polymers: These release antimicrobial chlorine dioxide gas when container headspace humidity reaches specific thresholds.
- Vapor-Phase Benzoxaboroles: Compounds that vaporize to provide uniform protection throughout the interior without requiring direct product contact.
Individual Film Wrapping and Mechanical Compression Integrity
Top Source Hygiene integrates specific structural formats to ensure that 100% Virgin Wood Pulp products maintain their factory-level sterility until they reach the end user. This multi-layered approach is critical for high-stacking logistics in 40HQ containers.
- Format A Individual Wrap: Each roll receives a dedicated primary barrier, serving as the gold standard for hospitality and premium retail hygiene.
- Mechanical Compression: Automated sealing technology minimizes bulk while completely isolating the product from environmental exposure during port handling.
- OBA-Free Standards: Packaging materials are strictly 100% OBA-Free to prevent the introduction of chemical sensitizers to the lint-free wood pulp.
- 5-Ply Export Cartons: Reinforced secondary protection prevents micro-tears in the primary film during the physical stress of transcontinental transit.
Why Is ISO 10993 Validation Essential for Skin Contact and Cytotoxicity?
ISO 10993 validation acts as a mandatory safety gatekeeper, using cytotoxicity and irritation tests to identify toxic leachables before tissue products reach sensitive consumers.
| ISO Standard | Primary Assessment | Safety Goal |
|---|---|---|
| ISO 10993-5 | Cytotoxicity Screening | Zero cell death at tissue interface |
| ISO 10993-23 | Skin Irritation (RhE models) | Prevention of inflammatory response |
| ISO 10993-10 | Sensitization Protocols | Detection of delayed allergic reactions |
Core Biocompatibility Endpoints for Hygiene Products
Standardized biological testing provides the data wholesalers need to guarantee product safety. We use ISO 10993-5 cytotoxicity testing as the primary tool to detect if extractables from the tissue cause cell death. This ensures that even prolonged contact with mucosal membranes remains safe.
- ISO 10993-23 Irritation: Technicians use reconstructed human epidermis models to evaluate potential inflammation during 18-24 hour exposure windows.
- ISO 10993-10 Sensitization: These protocols determine if repeated contact triggers delayed hypersensitivity or specific allergic reactions over time.
- Objective Scoring: Laboratories apply grades (0-4) to provide empirical data for risk mitigation during the initial material selection phase.
Safety Validation of OBA-Free Virgin Wood Pulp
Material purity is the first line of defense against cytotoxicity. At Top Source Hygiene, we source 100% Virgin Wood Pulp from the Hebei industrial hub to maintain long-fiber integrity. This specific selection reduces lint-related irritation that often compromises the skin barrier in lower-grade products.
- OBA-Free Guarantee: We eliminate Optical Brightening Agents (OBAs) to prevent the risk of fluorescent-induced skin irritation for sensitive users.
- Natural Brightness: Our fiber selection achieves 85-92% brightness naturally, avoiding aggressive chemical bleaching that could trigger cytotoxic responses.
- Contaminant Control: Compliance with FDA and EU hygiene standards ensures no recycled waste contaminants are present in the final roll.
- Fiber Integrity: High-purity wood pulp maintains a lint-free experience, which is critical for medical and hospitality environments where hygiene is non-negotiable.

Can Core Treatment Prevent Pathogen Growth Inside the Roll?
Antimicrobial coatings and high-purity virgin pulp prevent internal pathogen colonies. These agents neutralize bacteria on the core surface while moisture-resistant barriers stop fungal growth during long-term storage.
Inhibiting Microbes Through Surface-Active Antimicrobial Coatings
We treat roll cores with active agents to turn a structural component into a hygiene barrier. Moisture often collects in the center of a roll during transit, making it a prime site for mold if left untreated.
- Bactericidal Surface: Application of silver ions and quaternary ammonium compounds to kill microbes on contact.
- Humidity Control: Incorporation of moisture-resistant additives to eliminate the damp environment fungal spores need to germinate.
- Chemical Stability: Long-term fungicides remain active throughout the product’s 2026 shelf life, protecting stock in tropical or humid warehouses.
These coatings ensure that even if the outer packaging suffers a micro-perforation, the internal structure of the roll remains a hostile environment for pathogen colonization.
Material Purity and ISO-Certified Barrier Compliance
Material choice determines the baseline risk. Recycled waste paper often carries a high microbial load from its previous lifecycle. We eliminate this risk by using 100% virgin wood pulp across our entire production line.
- 100% Virgin Wood Pulp: Using primary fibers avoids the microbial risks inherently found in recycled waste paper cores.
- Global Standards: Compliance with FDA and EU hygiene protocols ensures all contact surfaces stay OBA-Free and non-toxic.
- Density Engineering: Models like the TSH-3396 utilize a high-density solid roll design that significantly reduces the internal surface area vulnerable to airborne contamination.
By combining high-purity fiber with dense winding, we limit the oxygen and space available for bacteria to move from the core to the actual usable tissue layers.
Frequently Asked Questions
How do certified laboratories test toilet paper for microbiological safety?
Technicians follow ISO 8784-1 and ISO 8784-3 protocols to determine the total colony-forming units (CFU) in pulp and paper samples. The process involves disintegrating the paper and culturing it at 20-25°C for aerobic bacteria and fungi. These tests specifically screen for pathogens like Salmonella and E. coli to ensure 100% virgin wood pulp is safe for consumer use.
Can factory-produced tissue rolls introduce bacteria into hospital environments?
Research shows hospital contamination typically happens during facility use rather than during manufacturing. While we produce factory-sealed rolls under high-heat conditions, environmental sources like sinks and jet air dryers in hospitals spread pathogens. Using individually wrapped rolls, such as the TSH-4010 luxury series, mitigates risks by protecting the paper until it is opened.
What is the acceptable microbial limit for medical-grade hygiene tissue?
No single global limit exists, but manufacturers adhere to USP <61> or European Pharmacopoeia standards. For products involving skin contact, laboratories often target a limit of 10² CFU/g. Validation through ISO 10993 for cytotoxicity remains a critical benchmark for products used in sensitive medical or clinical settings.
How does specialized packaging maintain sterility during international shipping?
Packaging systems use bacterial barrier materials and reinforced seals to prevent microbial intrusion. During transcontinental transport in 40HQ containers, we use secondary protection like 5-ply export cartons and mechanical compression technology to ensure seal integrity. This prevents contamination from moisture or dust while maintaining paper softness.
Does bamboo paper provide natural antibacterial benefits compared to wood pulp?
Bamboo fiber naturally contains a bio-agent called bamboo kun, which provides bacteriostatic effects. Laboratory tests show bamboo tissues can reduce bacterial presence by 70% compared to standard wood pulp. While Top Source Hygiene primarily uses 100% virgin wood pulp for strength, we provide bamboo options for projects requiring natural antimicrobial properties.
Why are standard facial tissues prohibited in sterile cleanrooms?
Conventional tissues shed thousands of microscopic fiber particles that violate ISO 14644 particulate standards. Cleanroom environments for medical device manufacturing require non-linting, certified alternatives that do not compromise air quality. Personnel must use approved, low-particle wipes in gowning areas to maintain required sterility levels.


