Sourcing toilet paper with Additives: Lotions & Scents is a critical risk management step for boutique retailers and medical procurement teams. Improperly stabilized emulsions or direct-sheet scenting often trigger contact dermatitis, resulting in rejected batches and damaged brand reputation.
This analysis assesses hygiene performance using the 100% OBA-free virgin wood pulp standard. We focus on 13–22g/m² ply density and point-to-point embossing to help you secure a hypoallergenic product line that balances skin safety with high-margin luxury appeal.
Why Is Skincare Infusion the Next Major Trend in Premium Hygiene?
2026 hygiene shifts toward barrier protection and microbiome safety. Premium buyers demand materials combining high-purity wood pulp with skin-soothing properties to prevent daily irritation.
The Growing Demand for Barrier-Focused Hygiene Surfaces
Hygiene in 2026 is moving beyond basic sanitation. Consumers now view every surface that touches their skin—including toilet paper and tissues—as part of their skincare routine. This shift forces a move away from abrasive, commodity-grade paper toward materials that protect the skin’s biological shield.
Wholesalers and hospitality groups are seeing a clear preference for surfaces that offer active wellness benefits rather than just cleaning utility. The market is prioritizing specific functional traits:
- Microbiome Sensitivity: Materials must prevent disruption to the skin’s natural barrier.
- Multifunctional Performance: Combining traditional strength with soothing, non-abrasive textures.
- Premium Positioning: Transitioning from bulk commodities to high-end wellness tools for luxury retail.
This trend allows distributors to command higher margins by offering products that cater to the “skin-conscious” demographic, where irritation-free daily routines are a non-negotiable requirement.
Safety Standards for OBA-Free Virgin Wood Pulp
Safety is the foundation of the skincare-hygiene crossover. To meet these new standards, Top Source Hygiene utilizes 100% Virgin Wood Pulp to eliminate the need for chemical additives. We ensure that the paper remains biologically “quiet” on the skin by strictly controlling the manufacturing specs.
True “skincare-grade” paper avoids the artificial brightness created by chemical bleaching. Instead, we rely on fiber selection and precise density control to achieve high performance without irritation:
- Chemical Safety: 100% OBA-Free (No Optical Brightening Agents) to protect sensitive skin types.
- Natural Brightness: Standardized at 85-92% through fiber purity rather than harsh bleaching.
- Ply Density: Strictly maintained at 13–22g/m² to balance high-wet-strength with a cloud-like feel.
- Fiber Integrity: Long-fiber wood pulp ensures a lint-free experience that won’t leave residue on sensitive areas.
By maintaining these technical parameters, we help B2B partners deliver a product that functions like a protective barrier. This technical approach removes the risks of contact dermatitis often associated with recycled or chemically enhanced paper products.
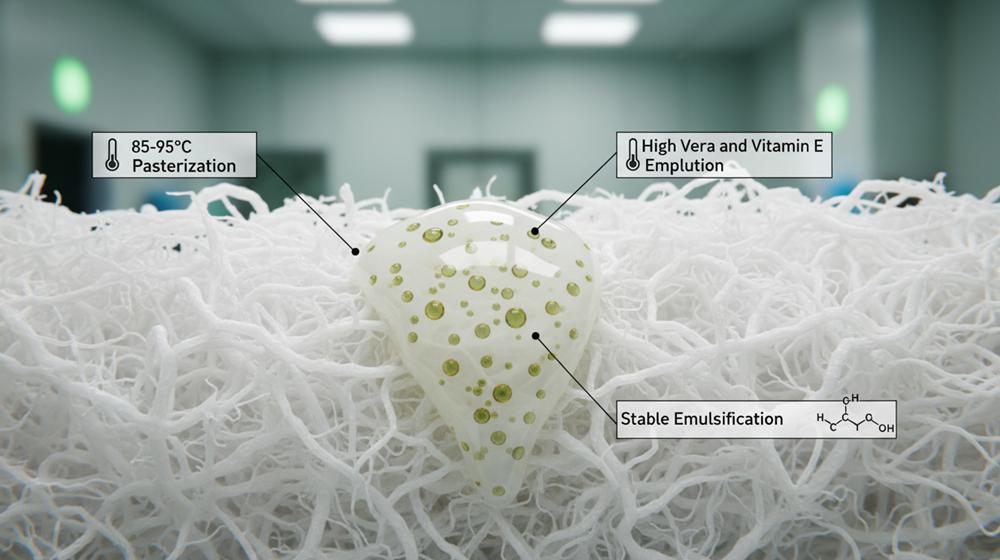
How Do We Formulate Stable Lotions Using Aloe Vera and Vitamin E?
Stable lotions require balancing aloe’s electrolytes with Vitamin E antioxidants through sequential mixing and 85-95°C pasteurization, ensuring skin-safe delivery via 100% virgin wood pulp fibers.
The Chemistry of Aloe and Vitamin E Emulsions
Aloe vera gel presents a unique challenge in industrial chemistry because its high electrolyte content often causes standard emulsions to separate. We solve this by building a foundation that pairs water-based bases with specific stabilizers to maintain a creamy, uniform texture throughout the production cycle.
- Emulsification: We stabilize the mixture using water-based foundations combined with quaternary ammonium salt emulsifiers.
- Electrolyte Management: Formulations include specific agents to prevent the high electrolyte levels in aloe vera from breaking the emulsion.
- Antioxidant Stability: We incorporate 99% pure di-alpha tocopherol acetate to serve as a stabilizing antioxidant for the entire batch.
- Thermal Processing: Labs execute sequential mixing at a precise 110°F to ensure a smooth, creamy consistency across high-volume production.
- Microbial Safety: We apply pasteurization between 85-95°C to eliminate microbial risks while protecting the integrity of the botanical nutrients.
Application on OBA-Free Virgin Wood Pulp
Once we stabilize the lotion, the application process must respect the integrity of the paper substrate. At Top Source Hygiene, we focus on 100% virgin wood pulp to ensure the lotion sits on a lint-free surface that won’t irritate the user or fall apart during use.
- Substrate Selection: We use 100% Virgin Wood Pulp to provide long-fiber strength and a lint-free surface for lotion application.
- Chemical Purity: We utilize OBA-free manufacturing to remove fluorescent whiteners that could react with lotion additives and irritate sensitive skin.
- Absorption Tech: Our point-to-point embossing increases the available surface area for lotion absorption by 30%.
- Ply Density: We maintain a density of 13–22g/m² per ply to support the weight of the lotion without losing wet-strength durability.
- Plumbing Compatibility: Finished products undergo testing to ensure they meet septic-safe standards for rapid disintegration in North American and European systems.
Build Your Premium Custom Toilet Paper Brand

Is Core-Scenting Safer Than Direct Sheet-Scenting for Sensitive Skin?
Core-scenting prevents direct chemical contact with skin by infusing the cardboard tube instead of the tissue fibers, eliminating the risk of fragrance-induced contact dermatitis.
Volatile Organic Compound Migration and Surface Contact
Direct sheet-scenting applies fragrance oils or synthetic perfumes directly onto the wood fibers. These additives often trigger contact dermatitis or localized irritation when the paper touches mucosal tissues. Core-scenting changes the delivery mechanism by using the cardboard tube as the primary carrier.
This method relies on ambient diffusion, allowing the aroma to move through the air without altering the chemical composition of the paper itself. By removing the direct fiber-to-skin transfer of scents, manufacturers can significantly lower the risk for users with known fragrance sensitivities or allergic predispositions.
100% OBA-Free Virgin Fiber Standards
Skin safety depends heavily on the base material. Even with indirect scenting, the fiber quality must remain high to prevent irritation. Top Source Hygiene utilizes 100% Virgin Wood Pulp to ensure a naturally lint-free surface, which prevents the micro-abrasions often caused by recycled alternatives.
- Chemical Integrity: Guaranteed 100% OBA-Free (No Optical Brightening Agents) production to protect the skin’s natural pH.
- Fiber Purity: Genuine long-fiber wood pulp provides high tensile strength without the need for harsh wet-strength additives.
- Compliance Standards: Materials meet ISO 9001 and EU hygiene standards for hypoallergenic applications.
Combining core-scented technology with high-purity virgin fibers creates a hypoallergenic environment. This approach is increasingly preferred in luxury hospitality and medical-grade retail markets where user safety is a non-negotiable requirement.

What Protocols Define a True “Hypoallergenic” Status in Manufacturing?
Hypoallergenic status relies on manufacturer-led protocols including OBA-free production and virgin wood pulp selection to ensure material purity and prevent irritation in high-volume supply chains.
| Validation Category | Standard/Protocol | Safety Outcome |
|---|---|---|
| Regulatory Baseline | FDA & EU Hygiene Standards | Verification of zero harmful chemical residues. |
| Material Purity | 100% OBA-Free Verification | Elimination of fluorescent brightening agents. |
| Fiber Performance | EN/IEC Performance Criteria | Consistency in lint-free, long-fiber structural integrity. |
| Process Control | ISO 9001 Batch Validation | Batch-to-batch stability across high-volume runs. |
Validating Safety through Industry Performance Standards
The FDA and other regulatory bodies do not provide a legal definition for the term “hypoallergenic” in consumer paper products. This lack of federal oversight means manufacturers must take the lead. We establish safety through industry benchmarks like EN, IEC, and AHAM criteria. These standards allow us to assess how materials interact with the skin under actual usage conditions.
Our Hebei production facility uses proprietary testing protocols to ensure that high-volume orders maintain their safety profile. We don’t just test the first roll; we validate the entire production run. This process requires documentation of material stability to prevent the formation of irritants during storage or transit.
- Standard Benchmarks: Utilization of EN/IEC/AHAM criteria to measure product safety objectively.
- Usage Protocols: Implementation of testing based on consumer contact frequency and allergen-specific criteria.
- Batch Validation: ISO 9001 consistency checks to maintain safety across 40HQ container volumes.
- Stability Records: Detailed monitoring of storage conditions to prevent material degradation.
Eliminating Irritants via OBA-Free Virgin Wood Pulp
True hypoallergenic status starts with the raw material. Most skin reactions in paper products stem from Optical Brightening Agents (OBAs) or contaminants found in recycled waste. We solve this by strictly using 100% Virgin Wood Pulp. This primary long-fiber material creates a lint-free experience that prevents micro-abrasions on sensitive tissue.
Our “100% OBA-Free” guarantee means we achieve 85-92% natural brightness through fiber selection rather than chemical bleaching. By removing these harsh additives, we meet both FDA and EU hygiene standards for skin-contact layers. The high-purity long fibers provide structural integrity without needing the chemical binders often found in lower-grade alternatives.
- OBA-Free Production: Zero fluorescent whiteners used, eliminating a primary source of contact dermatitis.
- 100% Virgin Wood Pulp: Premium long-fiber selection ensures a lint-free surface and high tensile strength.
- Regulatory Compliance: Strict adherence to FDA and EU hygiene standards for all skin-contact materials.
- Material Integrity: High-purity fibers maintain durability without relying on harsh chemical binders.
Do Chemical Additives Compromise Paper Absorbency or Flushability?
Sizing and wet-strength agents limit water absorption by design, but mechanical embossing and 100% virgin pulp maintain absorbency and rapid disintegration for plumbing safety.
Functional Impacts of Sizing and Wet-Strength Agents
Chemical additives serve specific structural roles but often create a trade-off with the paper’s ability to pull in moisture. Manufacturers use these agents to ensure the sheet doesn’t fall apart the moment it touches liquid, though over-application can lead to a noticeable drop in performance for the end-user.
- Sizing Agents: Chemicals like alkyl ketene dimer (AKD) and rosin impart waterproofing properties. These help the paper maintain its structural integrity during printing or use.
- Wet-Strength Polymers: These create water-resistant coatings on cellulose fibers. Research indicates these can reduce water absorption capacity by up to 35% compared to untreated paper.
- Retention Aids: Polyacrylamides allow us to adjust paper porosity. This allows for tailoring the sheet for either high-absorbency tissue or smooth, dense packaging.
- Fiber Integrity: Calibrated chemical applications prevent the paper from becoming “linty” or shedding fibers, ensuring a cleaner experience.
Enhancing Absorbency Through Point-to-Point Embossing
To counter the absorbency loss caused by necessary chemicals, we utilize mechanical engineering. By changing the physical structure of the paper, we restore the “thirsty” quality required for premium hygiene products without adding more chemical load.
- Point-to-Point Embossing: This CNC-precision technique creates air pockets between plies, physically increasing liquid absorbency by 30% without chemical intervention.
- 100% Virgin Wood Pulp: Using long-fiber wood pulp from our Hebei facility ensures high tensile strength. This material is 100% OBA-Free, meaning no fluorescent whiteners touch the skin.
- Rapid Disintegration: We engineer the fiber bonds to dissolve quickly in water. This ensures the paper meets international septic-safe standards and prevents plumbing clogs.
- Precision Perforation: Clean-cut lines ensure the paper tears exactly where intended. This prevents the “shredded edges” common in lower-grade recycled products with imbalanced bonding.

How to Ensure Global Regulatory Compliance Under FDA and REACH?
Compliance requires aligning with FDA MoCRA and REACH. Leading manufacturers ensure safety through 100% OBA-free material selection, INCI ingredient disclosure, and rigorous batch-specific chemical testing.
Global markets demand strict adherence to chemical safety standards. Importers must verify that every additive, from scents to moisturizing lotions, meets the evolving legal requirements of the target region.
Regulatory Frameworks for Scent and Lotion Additives
The FDA MoCRA guidelines mandate facility registration and full supply chain traceability for all pigments and lotion components. You cannot bypass these requirements if you intend to distribute in the United States.
- INCI Naming: Ingredient disclosure protocols require listing all substances using International Nomenclature of Cosmetic Ingredients naming conventions.
- Safety Substantiation: Factories must maintain validated assessments, Certificates of Analysis (CoA), and testing results for heavy metals.
- REACH Compliance: European Union regulations necessitate substance registration and hazard classification for all chemical additives used in the manufacturing process.
- Traceability: Every cosmetic pigment must be traceable back to the original manufacturer, including intermediate processing steps.
Adopting OBA-Free and ISO 9001 Manufacturing Protocols
Top Source Hygiene utilizes 100% OBA-free virgin wood pulp. This eliminates fluorescent whitening agents and satisfies the specific safety demands of both European and North American distributors.
- Material Purity: We use 100% Virgin Wood Pulp to prevent cross-contamination from recycled waste, ensuring a lint-free and chemically pure product.
- Quality Control: ISO 9001 certified systems monitor fiber integrity and chemical application consistency across high-volume 40HQ container orders.
- Audit Readiness: Detailed manufacturing records and pre-shipment visual verification provide the transparency required for successful customs clearance.
- Septic Safety: Our engineering ensures paper dissolves quickly to meet international plumbing standards while maintaining high wet-strength during use.
We strictly protect your cash flow and regulatory standing. By providing full documentation and loading videos before balance payments, we ensure your 40HQ shipment meets every regional safety protocol without delay.
Frequently Asked Questions
Is scented toilet paper safe for users with chronic skin sensitivities?
Health experts advise against scented products for chronic sensitivities. Chemicals in fragrances and dyes often disrupt genital pH and trigger contact dermatitis or infections. For these users, Top Source Hygiene recommends OBA-free, 100% virgin wood pulp options to eliminate chemical irritants and protect skin integrity.
What are the most effective additives for creating a premium lotion feel?
Premium formulations rely on moisturizing agents like Aloe Vera and Hyaluronic Acid to hydrate the skin. Softening agents such as Dimethicone and fatty acid esters create a silky, low-friction texture. Adding Vitamin E or Chamomile extract provides additional antioxidant and anti-inflammatory benefits for irritated skin.
How do manufacturers prevent scents from triggering allergic reactions?
Safety is managed through strict toxicological evaluations and compliance with International Fragrance Association (IFRA) standards. Manufacturers use patch testing on human subjects and maintain detailed Safety Data Sheets (SDS) to monitor aggregate exposure and ensure all fragrance compounds remain within safe clinical limits.
Does adding moisturizing lotion make the paper less absorbent?
Modern tissue engineering prevents absorbency loss by using targeted lotion placement rather than uniform fiber coating. By applying cream-based emulsions to surface layers or using discrete deposits, the paper maintains its hydrophilic pathways, allowing moisture to penetrate the core while the lotion remains on the surface.
What protocols define a true dermatologically tested status?
This designation requires controlled patch testing on at least 30 human subjects. A dermatologist monitors the skin for 24 to 48 hours to evaluate irritation against a water control. A ‘Very Good’ rating is achieved when fewer than 10% of subjects show even slight reactions during the clinical study.
Can botanical-based scents meet B2B safety and ESG requirements?
Botanical scents meet global standards when verified for IFRA compliance and proper dilution. To align with ESG goals, suppliers provide traceability documentation and sustainability certifications. This ensures natural extracts like lavender or rose oil are harvested ethically and processed without compromising user safety.
Final Thoughts
Relying on low-grade recycled fibers or chemically bleached paper risks brand erosion and liability through contact dermatitis claims. Investing in 100% OBA-free virgin wood pulp secures your margin by meeting the non-negotiable safety standards of premium medical and luxury retail markets. This technical shift from commodity hygiene to skin-safe wellness tools transforms a basic necessity into a high-retention asset for your distribution network.
Eliminate guesswork by requesting a technical specification sheet or a sample kit to verify the 30% absorbency increase of our point-to-point embossing. Our Hebei facility supports your private label transition with 40HQ trial orders that maximize container payload and minimize landed costs. Contact our team today to discuss lead times and secure a production slot for your custom-branded hygiene line.


